Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
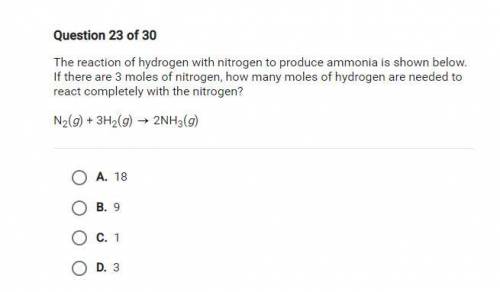
The reaction of hydrogen with nitrogen to produce ammonia is shown below. If there are 3 moles of ni...
Questions

History, 29.09.2019 11:30

Mathematics, 29.09.2019 11:30

History, 29.09.2019 11:30



English, 29.09.2019 11:30

Social Studies, 29.09.2019 11:30

Chemistry, 29.09.2019 11:30





Mathematics, 29.09.2019 11:30


Biology, 29.09.2019 11:30


Mathematics, 29.09.2019 11:30


Mathematics, 29.09.2019 11:30

History, 29.09.2019 11:30