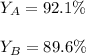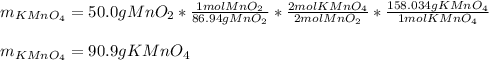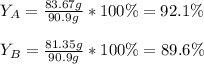Scientist A produces 83.67 g KMnO4 while Scientist B produces 81.35 g KMnO4.
What is the percent yield for Scientist A?
What is the percent yield for Scientist B?
You must show all work to receive full credit.
The equation for the production of potassium permanganate is as follows:
2 MnO2 + 2 KOH + O2 → 2 KMnO4 + H2

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Scientist A produces 83.67 g KMnO4 while Scientist B produces 81.35 g KMnO4.
What is the percent yi...
Questions

Biology, 16.10.2019 04:00

English, 16.10.2019 04:00


English, 16.10.2019 04:00

Chemistry, 16.10.2019 04:00

Computers and Technology, 16.10.2019 04:00

Mathematics, 16.10.2019 04:00







Mathematics, 16.10.2019 04:00

Mathematics, 16.10.2019 04:00

Mathematics, 16.10.2019 04:00


History, 16.10.2019 04:00