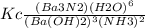Chemistry, 07.06.2021 22:50 teddybear196510
The expression for the equilibrium constant for the reaction:
Ba3N2 (aq) + 6 H2O(1)-
3 Ba(OH)2 (aq) + 2 NH3 (9)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
The expression for the equilibrium constant for the reaction:
Ba3N2 (aq) + 6 H2O(1)-
3 Ba(OH)...
3 Ba(OH)...
Questions


Mathematics, 02.11.2020 22:10

Physics, 02.11.2020 22:10


History, 02.11.2020 22:10


Health, 02.11.2020 22:10

Business, 02.11.2020 22:10



Mathematics, 02.11.2020 22:10


Geography, 02.11.2020 22:10



Mathematics, 02.11.2020 22:10

Mathematics, 02.11.2020 22:10

Mathematics, 02.11.2020 22:10

History, 02.11.2020 22:10