
Chemistry, 07.06.2021 22:50 naomicervero
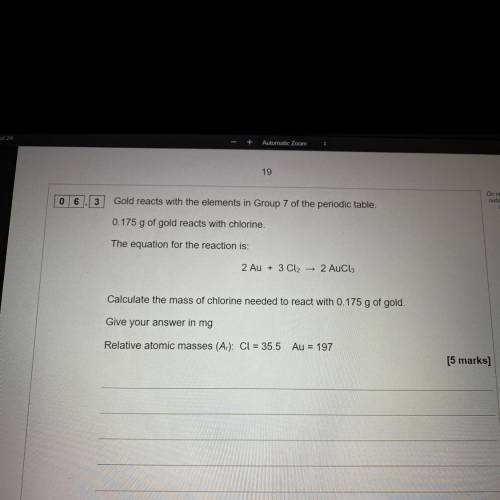
Gold reacts with elements in group 7 of the periodic table 0.175g of gold reacts with chlorine.
The equation for reaction for the reaction is
2Au + 3cl2 ——> 2Aucl3
Calculate the mass of chlorine needed to react with 0.175g of gold. Give your answer in mg
Relative atomic masses cl=35.5 Au=197

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Gold reacts with elements in group 7 of the periodic table 0.175g of gold reacts with chlorine.
The...
Questions

Mathematics, 17.08.2020 23:01






Mathematics, 17.08.2020 23:01

Mathematics, 17.08.2020 23:01



English, 17.08.2020 23:01


Mathematics, 17.08.2020 23:01




Mathematics, 18.08.2020 01:01


Mathematics, 18.08.2020 01:01

Mathematics, 18.08.2020 01:01



