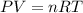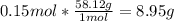Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
A) If we have a 4.5 L container of CH 10 gas at a temperature of 178 K and a pressure of 0.50 atm, t...
Questions


Mathematics, 15.06.2021 18:10


Computers and Technology, 15.06.2021 18:10



Geography, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10




Mathematics, 15.06.2021 18:10

Biology, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10

Mathematics, 15.06.2021 18:10

Biology, 15.06.2021 18:10


Chemistry, 15.06.2021 18:10