Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2

Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2
You know the right answer?
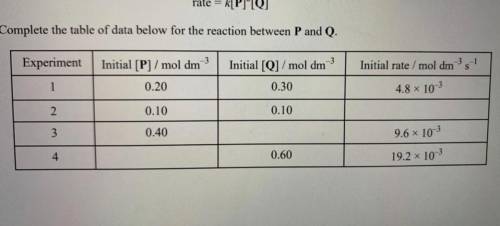
The initial rate of the reaction between substances P and Q was measured in a series of
experiments...
Questions




Mathematics, 20.04.2020 22:35











Chemistry, 20.04.2020 22:35

Health, 20.04.2020 22:35

Mathematics, 20.04.2020 22:35


Mathematics, 20.04.2020 22:35

Law, 20.04.2020 22:35