
Chemistry, 08.06.2021 16:40 AbigailHaylei
4Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
A. What is the oxidation number for each item in this equation?
B. What is being oxidized?
C. What is being reduced?
D. What is the oxidizing agent?
E. What is the reducing agent?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
4Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
A. What is the oxidation number for each item in this equation?
Questions







Mathematics, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00


Mathematics, 27.10.2020 23:00


Mathematics, 27.10.2020 23:00





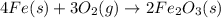
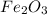 is +3.
is +3. 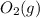 is 0 as it is present in its elemental state.
is 0 as it is present in its elemental state.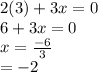
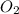 as a decrease in its oxidation state is occurring. So,
as a decrease in its oxidation state is occurring. So, 


