
Chemistry, 08.06.2021 17:10 dogerboy8380
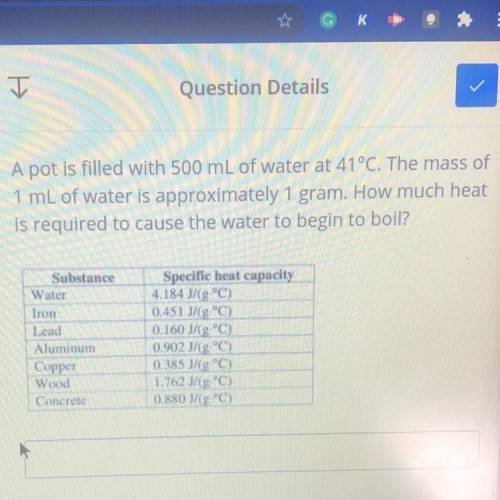
A pot is filled with 500 mL of water at 41°C. The mass of
1 mL of water is approximately 1 gram. How much heat
is required to cause the water to begin to boil?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

You know the right answer?
A pot is filled with 500 mL of water at 41°C. The mass of
1 mL of water is approximately 1 gram. Ho...
Questions

Mathematics, 05.10.2020 02:01








Mathematics, 05.10.2020 02:01




Computers and Technology, 05.10.2020 02:01

Mathematics, 05.10.2020 02:01

Mathematics, 05.10.2020 02:01


English, 05.10.2020 02:01

Mathematics, 05.10.2020 02:01




