Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
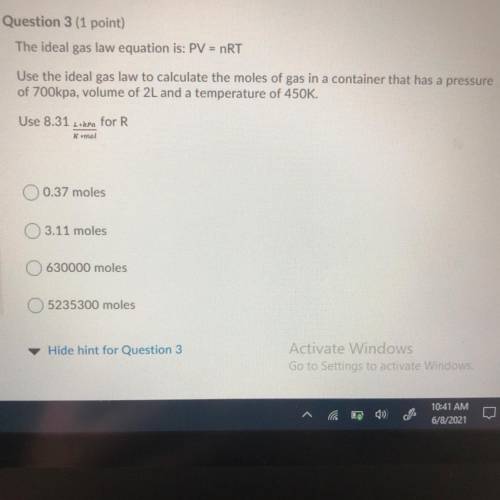
The ideal gas law equation is: PV = nRT
Use the ideal gas law to calculate the moles of gas in a co...
Questions


History, 04.08.2019 19:30


Biology, 04.08.2019 19:30

History, 04.08.2019 19:30




Mathematics, 04.08.2019 19:30




Business, 04.08.2019 19:30



Physics, 04.08.2019 19:30


Social Studies, 04.08.2019 19:30

Social Studies, 04.08.2019 19:30

Biology, 04.08.2019 19:30