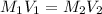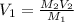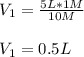Question 8 (5 points)
(08.02 MC)
A 10 M concentrated stock solution of NaCl was used to prepa...

Chemistry, 08.06.2021 20:50 ELGuapo6746
Question 8 (5 points)
(08.02 MC)
A 10 M concentrated stock solution of NaCl was used to prepare 5 liters of diluted 1 M solution. Which of the following statements is true about
the
process used to achieve this required dilution? (5 points)
O a
The volume of stock solution used was less than 0.4 liters.
Oь
The volume of stock solution used was more than 5 liters.
Ос
The volume of the solvent used was less than 0.4 liters.
Od
The volume of the solvent used was less than 5 liters.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1


Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
Questions


History, 10.11.2020 07:00


Biology, 10.11.2020 07:00


Mathematics, 10.11.2020 07:00

Social Studies, 10.11.2020 07:00

Mathematics, 10.11.2020 07:00



English, 10.11.2020 07:00



Biology, 10.11.2020 07:00



Mathematics, 10.11.2020 07:00


Mathematics, 10.11.2020 07:00

Computers and Technology, 10.11.2020 07:00