Considering the following intermediate chemical equations. 2H2(g)+O2(g) 2H2O(I)
...

Chemistry, 08.06.2021 21:10 historygal
Considering the following intermediate chemical equations. 2H2(g)+O2(g) 2H2O(I)
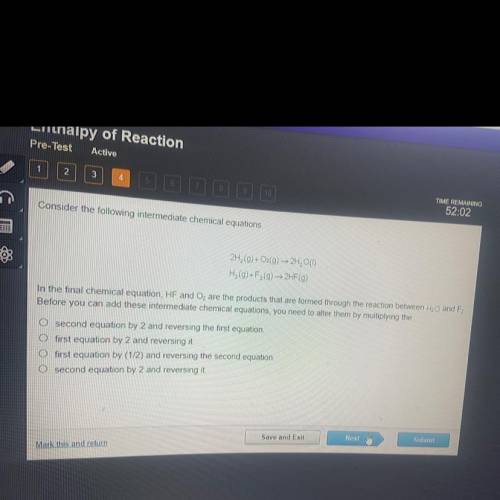

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Questions






Biology, 09.10.2020 04:01


English, 09.10.2020 04:01




History, 09.10.2020 04:01

Computers and Technology, 09.10.2020 04:01

History, 09.10.2020 04:01


History, 09.10.2020 04:01


Business, 09.10.2020 04:01

Chemistry, 09.10.2020 04:01



