
Chemistry, 09.06.2021 01:20 erik1franks
You combine 0.75 moles formate and 0.85 moles formic acid to make a buffer solution. The Ka of formic acid is 1.8x10-4 what is the pH of the solution

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
You combine 0.75 moles formate and 0.85 moles formic acid to make a buffer solution. The Ka of formi...
Questions




Mathematics, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31


Physics, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31


Mathematics, 29.10.2019 22:31



Mathematics, 29.10.2019 22:31


Social Studies, 29.10.2019 22:31

Computers and Technology, 29.10.2019 22:31


Biology, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31

Health, 29.10.2019 22:31

![\frac{[Formate]}{[Formic Acid]}](/tpl/images/1367/7318/f74f8.png) Where pKa = -log(Ka)pKa = -log(1.8x10⁻⁴) = 3.74
Where pKa = -log(Ka)pKa = -log(1.8x10⁻⁴) = 3.74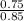 pH = 3.68
pH = 3.68


