Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
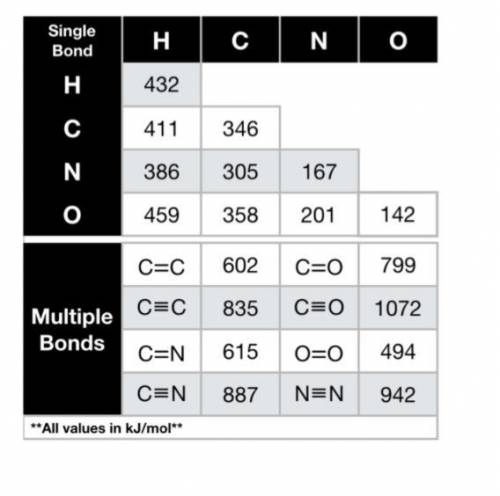
Using the bond energies provided, calculate the enthalpy of the reaction (∆Hrxn, in kJ) for the comb...
Questions

Computers and Technology, 12.12.2021 21:30

Mathematics, 12.12.2021 21:30

Mathematics, 12.12.2021 21:30



Mathematics, 12.12.2021 21:30

Computers and Technology, 12.12.2021 21:30


Mathematics, 12.12.2021 21:30



English, 12.12.2021 21:30

Computers and Technology, 12.12.2021 21:30

Social Studies, 12.12.2021 21:30


Social Studies, 12.12.2021 21:30



Arts, 12.12.2021 21:30