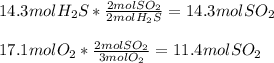Chemistry, 09.06.2021 07:50 Miloflippin7339
How much energy is used when 14.3 moles of hydrosulfuric acid reacts with 17.1 moles
of oxygen?
2 H2S + 3 02 + 175 KJ
—->2 SO2 + 2 H20
Which substance is the limiting reactant?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

You know the right answer?
How much energy is used when 14.3 moles of hydrosulfuric acid reacts with 17.1 moles
of oxygen?
Questions


Mathematics, 27.08.2019 04:30

English, 27.08.2019 04:30


Computers and Technology, 27.08.2019 04:30

Arts, 27.08.2019 04:30

History, 27.08.2019 04:30

English, 27.08.2019 04:30

History, 27.08.2019 04:30

History, 27.08.2019 04:30

Biology, 27.08.2019 04:30



Mathematics, 27.08.2019 04:30

Physics, 27.08.2019 04:30

History, 27.08.2019 04:30



Mathematics, 27.08.2019 04:30

Mathematics, 27.08.2019 04:30