Chemistry, 09.06.2021 08:50 bbenaventbbbb9653
Enough nitrogen must be generated in the bag to create a total pressure of 2.56 atm, which then drops as the bag slowly deflates. Assuming the volume of the bag is 56.0 L and the temperature in the car is 25oC, calculate the molar quantity (number of moles) of N2 that must be generated. Hint: Use Ideal gas law PV=nRT (Ideal gas constant R=0.080 L. atm/mol. K)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3



Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Enough nitrogen must be generated in the bag to create a total pressure of 2.56 atm, which then drop...
Questions


Mathematics, 29.11.2021 20:30


Biology, 29.11.2021 20:30

Chemistry, 29.11.2021 20:30



History, 29.11.2021 20:30


Mathematics, 29.11.2021 20:30

English, 29.11.2021 20:30

Mathematics, 29.11.2021 20:30

Mathematics, 29.11.2021 20:30


Mathematics, 29.11.2021 20:30

Health, 29.11.2021 20:30



Mathematics, 29.11.2021 20:30

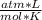 T= 25 C= 298 K (being 0 C= 273 K)
T= 25 C= 298 K (being 0 C= 273 K)