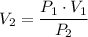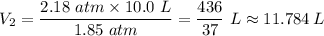Chemistry, 09.06.2021 18:00 Hailey1313131313
your car tire is filled with air at a pressure of 2.18 atm and have a volume of 10.0L. After riding around for a couple month, your tire pressure goes down to 1.85 atm. what is new volume of your tire

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 2


Chemistry, 23.06.2019 06:40
1.) which of the following is a molecule but not a compound? a.he b.f2 c.h2o d.ch4 2.) what is a physical combination of substances? a.a compound b.a molecule c.a mixture d.an element 3.) what is a chemical combination of substances? a.a compound b.an atom c.a mixture d.an element 4.) what is the relationship between the solute and solvent in a solution? a.they form a compound b.they form a mixture c.they form molecules d.they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a.add more water b.reduce the air pressure c.increase the air pressure d.stir the water 6.) how would you determine the solubility of a substance? a.find how well it dissolved various substances. b.find the mass and the volume of the substance. c.find the temperature at which the substance evaporated. d.find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a.molecules. b.compounds. c.atoms. d.ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a.whether they can be easily separated b.whether they chemically bond together c.whether they both are visible d.whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a.molecules and atoms. b.molecules. c.compounds and molecules. d.atoms.
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
your car tire is filled with air at a pressure of 2.18 atm and have a volume of 10.0L. After riding...
Questions

Mathematics, 06.01.2021 18:50

English, 06.01.2021 18:50

Mathematics, 06.01.2021 18:50



English, 06.01.2021 18:50




Mathematics, 06.01.2021 18:50


History, 06.01.2021 18:50

History, 06.01.2021 18:50

Chemistry, 06.01.2021 18:50


Mathematics, 06.01.2021 18:50

History, 06.01.2021 18:50


Mathematics, 06.01.2021 18:50

History, 06.01.2021 18:50