
Chemistry, 10.06.2021 01:00 adanaguirre17
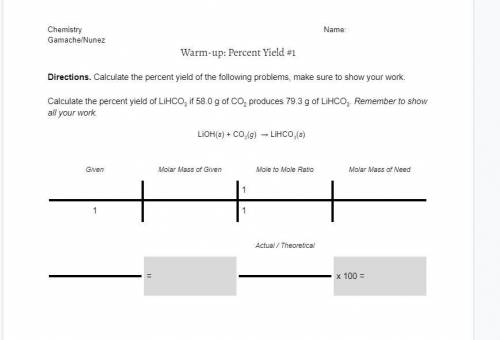
Directions. Calculate the percent yield of the following problems, make sure to show your work.
Calculate the percent yield of LiHCO3 if 58.0 g of CO2 produces 79.3 g of LiHCO3. Remember to show all your work.
LiOH(s) + CO2(g) → LiHCO3(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1


Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Directions. Calculate the percent yield of the following problems, make sure to show your work.
Ca...
Questions



English, 04.10.2020 08:01

History, 04.10.2020 08:01


English, 04.10.2020 08:01


Mathematics, 04.10.2020 08:01




Mathematics, 04.10.2020 08:01

Arts, 04.10.2020 08:01

Mathematics, 04.10.2020 08:01

Spanish, 04.10.2020 08:01

Biology, 04.10.2020 08:01



Mathematics, 04.10.2020 08:01

Mathematics, 04.10.2020 08:01



