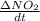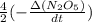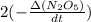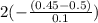Chemistry, 10.06.2021 03:50 tatibean26
If the initial concentration of N2O5 was 0.500 M and the concentration of N2O5 was 0.450 M after 0.100 s, what is the average rate of NO2 formation during the first 100 milliseconds

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
If the initial concentration of N2O5 was 0.500 M and the concentration of N2O5 was 0.450 M after 0.1...
Questions

History, 24.10.2019 03:20


English, 24.10.2019 03:20

Social Studies, 24.10.2019 03:20

Mathematics, 24.10.2019 03:20


Social Studies, 24.10.2019 03:20




Mathematics, 24.10.2019 03:20

Health, 24.10.2019 03:20

Mathematics, 24.10.2019 03:20

Mathematics, 24.10.2019 03:20

Chemistry, 24.10.2019 03:20


Biology, 24.10.2019 03:20


Chemistry, 24.10.2019 03:20

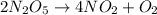
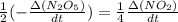
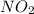 formation:
formation: