
Chemistry, 10.06.2021 14:10 sherlock19
The Copper Chloride solution used in the investigation contained 300 grams per dm3 of solid CuCl2 dissolved in 1dm3 of water. The student used 50cm3 of copper chloride solution in each experiment. Calculate the mass of solid copper chloride used in each experiment.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
The Copper Chloride solution used in the investigation contained 300 grams per dm3 of solid CuCl2 di...
Questions


Mathematics, 04.03.2021 19:00


Spanish, 04.03.2021 19:00




Chemistry, 04.03.2021 19:00





Mathematics, 04.03.2021 19:00




Chemistry, 04.03.2021 19:00



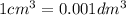
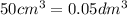
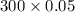 g
g

