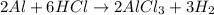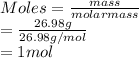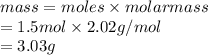Chemistry, 10.06.2021 15:20 drandbone92
Calculate the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid according to the following balanced chemical equation: 2 Al + 6 HCl → 2 AlCl3 + 3 H2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Calculate the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid...
Questions




Mathematics, 31.07.2019 03:30

Biology, 31.07.2019 03:30





History, 31.07.2019 03:30

Biology, 31.07.2019 03:30






English, 31.07.2019 03:30

Mathematics, 31.07.2019 03:30