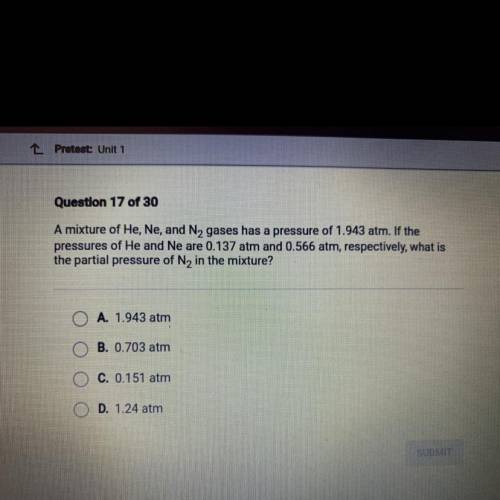
Question 17 of 30
A mixture of He, Ne, and N, gases has a pressure of 1.943 atm. If the
press...

Chemistry, 10.06.2021 19:30 babyambs50
Question 17 of 30
A mixture of He, Ne, and N, gases has a pressure of 1.943 atm. If the
pressures of He and Ne are 0.137 atm and 0.566 atm, respectively, what is
the partial pressure of N2 in the mixture?
A. 1.943 atm
B. 0.703 atm
C. 0.151 atm
D. 1.24 atm
SUBMIT

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Questions


Mathematics, 15.06.2021 22:10



Mathematics, 15.06.2021 22:10




History, 15.06.2021 22:10













