
Chemistry, 10.06.2021 20:30 giusto1073
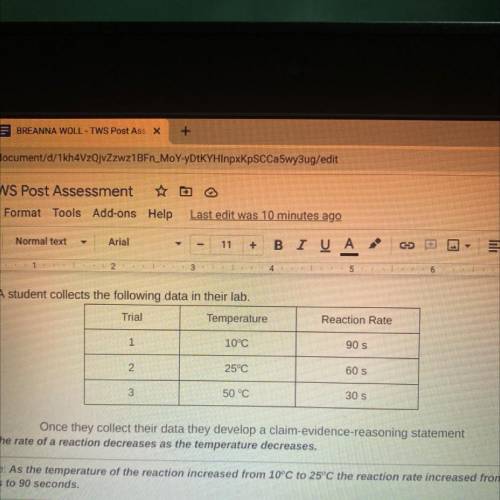
8. A student collects the following data in their lab.
Trial
Temperature
Reaction Rate
Once they collect their data they develop a claim-evidence-reasoning statement
Claim: The rate of a reaction decreases as the temperature decreases.
Evidence: As the temperature of the reaction increased from 10°C to 25°C the reaction rate increased from 60
seconds to 90 seconds.
Reasoning: Reaction rate depends on the temperature of the reactants. So when the temperature decreases, so
should the reaction rate. Particles need to collide in order to react. Lower temperatures allow molecules to move
slow enough to stick together and react so the reaction rate decreases.
Use the space below to provide feedback to the student. Was their conclusion correct? How should they fix it?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
8. A student collects the following data in their lab.
Trial
Temperature
Reaction Rate<...
Temperature
Reaction Rate<...
Questions


Mathematics, 10.03.2021 16:50

Mathematics, 10.03.2021 16:50


Mathematics, 10.03.2021 16:50






Computers and Technology, 10.03.2021 16:50

History, 10.03.2021 16:50


English, 10.03.2021 16:50

Mathematics, 10.03.2021 16:50







