
Chemistry, 10.06.2021 21:30 xxgissellexx
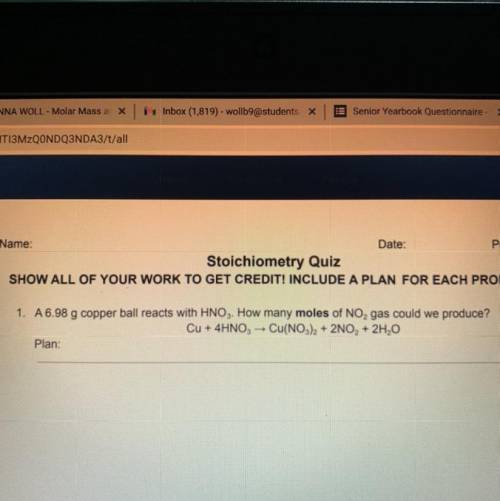
A 6.98 g copper ball reacts with HNO3. How many moles of NO, gas could we produce? Cu + 4HNO3 → Cu(NO3)2 + 2NO, + 2H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
A 6.98 g copper ball reacts with HNO3. How many moles of NO, gas could we produce?
Cu + 4HNO3 → Cu(...
Questions

Mathematics, 06.04.2021 16:50



Mathematics, 06.04.2021 16:50




Mathematics, 06.04.2021 16:50



Physics, 06.04.2021 16:50

Social Studies, 06.04.2021 16:50

Chemistry, 06.04.2021 16:50

Mathematics, 06.04.2021 16:50

Mathematics, 06.04.2021 16:50


Mathematics, 06.04.2021 16:50

Mathematics, 06.04.2021 16:50





