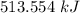Chemistry, 10.06.2021 21:50 Imamdiallo18
Given the equation 2Si(s) + 2Cl2(g) --> 2SiCl2(g) + 687 kJ, how much heat is produced when 106 g of Cl2 react?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

You know the right answer?
Given the equation 2Si(s) + 2Cl2(g) --> 2SiCl2(g) + 687 kJ, how much heat is produced when 106 g...
Questions


Social Studies, 08.01.2021 14:00

Social Studies, 08.01.2021 14:00

Mathematics, 08.01.2021 14:00

Geography, 08.01.2021 14:00


English, 08.01.2021 14:00


Arts, 08.01.2021 14:00

Mathematics, 08.01.2021 14:00

Mathematics, 08.01.2021 14:00

Computers and Technology, 08.01.2021 14:00

Physics, 08.01.2021 14:00

World Languages, 08.01.2021 14:00

Mathematics, 08.01.2021 14:00




Mathematics, 08.01.2021 14:00

Chemistry, 08.01.2021 14:00

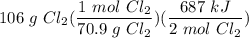 [DA] Divide/Multiply [Cancel out units]:
[DA] Divide/Multiply [Cancel out units]: