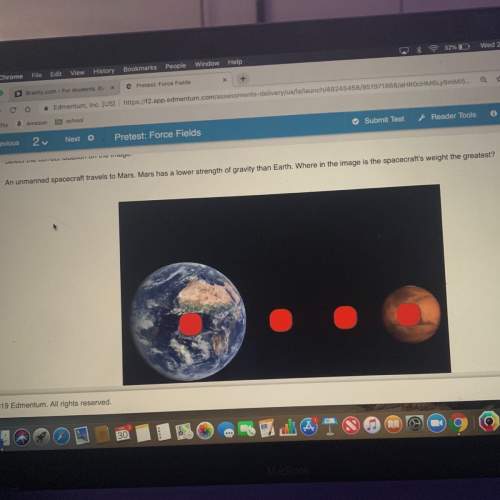
Chemistry, 10.06.2021 22:40 powella033
How many moles of aluminum oxide are produced according to the reaction below given that you start with 40.0 grams of Al and 19.0 grams of O2?
Reaction: 4Al + 3O2 --> 2Al2O3
A. 0.185
B. 0.741
C. 5.00
D. 0.396

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
How many moles of aluminum oxide are produced according to the reaction below given that you start w...
Questions

Mathematics, 14.09.2021 14:00



Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00







Mathematics, 14.09.2021 14:00


Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00


Chemistry, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00