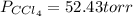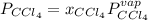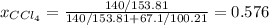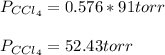At a certain temperature the vapor pressure of pure chloroform (CHCl3) is measured to be 91. torr. Suppose a solution is prepared by mixing 140. g of chloroform and 67.1 g of heptane (C, H16) of chloroform and 67.1 g of heptane (C7H16 Calculate the partial pressure of chloroform vapor above this solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
At a certain temperature the vapor pressure of pure chloroform (CHCl3) is measured to be 91. torr. S...
Questions


History, 04.11.2020 19:00

History, 04.11.2020 19:00


Geography, 04.11.2020 19:00

Mathematics, 04.11.2020 19:00









Chemistry, 04.11.2020 19:00