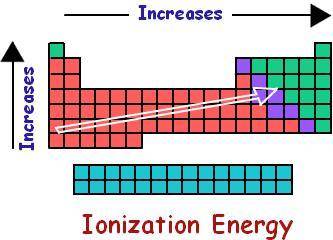
The first ionization energy of an element is the energy required to remove an electron from a gaseous atom of an element to produce a +1 ion:
M(g) + energy ---> M ^(+) (g) + e -
How do you think the activity of an element ought to be related to its first ionization energy? Predict a decreasing order of reactivity of the above elements based on their first ionization energies.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
The first ionization energy of an element is the energy required to remove an electron from a gaseou...
Questions

Mathematics, 09.02.2021 03:10

Social Studies, 09.02.2021 03:10

Advanced Placement (AP), 09.02.2021 03:10

Mathematics, 09.02.2021 03:10

Social Studies, 09.02.2021 03:10


Mathematics, 09.02.2021 03:10



Mathematics, 09.02.2021 03:10









English, 09.02.2021 03:10

Chemistry, 09.02.2021 03:10