
Chemistry, 12.06.2021 01:10 kelyanthecrafte
Help me ASAP PLS
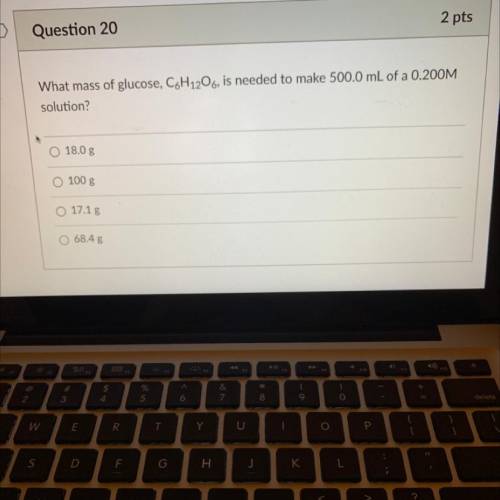
What mass of glucose, C6H12O6, is needed to make 500.0 mL of a 0.200M
solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Help me ASAP PLS
What mass of glucose, C6H12O6, is needed to make 500.0 mL of a 0.200M
soluti...
soluti...
Questions


Mathematics, 28.11.2019 04:31



Computers and Technology, 28.11.2019 04:31







Computers and Technology, 28.11.2019 04:31





Computers and Technology, 28.11.2019 04:31





