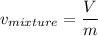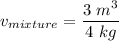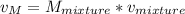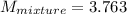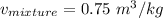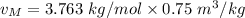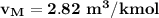Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
2 kg of hydrogen (H2) is mixed with 2 kg of oxygen (O2). If the final mixture has a volume of 3 m3:...
Questions

English, 10.05.2021 21:50


Mathematics, 10.05.2021 21:50




Mathematics, 10.05.2021 21:50

English, 10.05.2021 21:50






Biology, 10.05.2021 21:50



Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50

Geography, 10.05.2021 21:50