
Chemistry, 12.06.2021 14:00 shetherealbrat
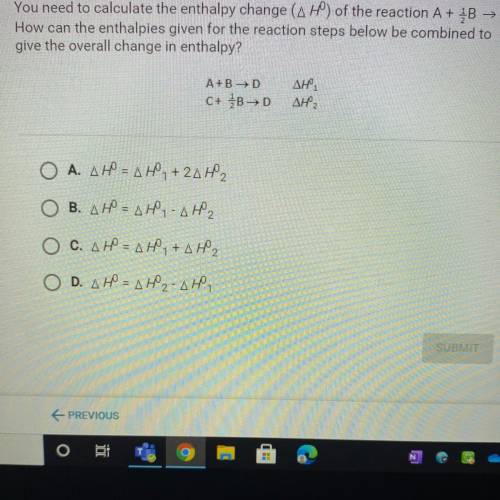
You need to calculate the enthalpy change (AH) of the reaction A + B → C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
A+B →D
C+ B>D
ΔΗ,
ΔΗ,
Ο Α. ΔΗ = ΔΗ, + 2ΔΗ,
B. AH = AH - AH2
O C. AHN AH,+AH2
O D. AH = AH2-AH,

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
You need to calculate the enthalpy change (AH) of the reaction A + B → C.
How can the enthalpies gi...
Questions








Mathematics, 16.07.2021 18:40








Chemistry, 16.07.2021 18:50






