
Chemistry, 12.06.2021 16:20 laiba012305
A 5.00 mL sample of vinegar, a solution of acetic acid, is titrated with 0.756 M calcium hydroxide. 10.23 mL of the calcium hydroxide is required to reach the equivalence point. What is the molarity of the acetic acid in the vinegar.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
A 5.00 mL sample of vinegar, a solution of acetic acid, is titrated with 0.756 M calcium hydroxide....
Questions


English, 12.10.2020 02:01

French, 12.10.2020 02:01

Chemistry, 12.10.2020 02:01

History, 12.10.2020 02:01

Computers and Technology, 12.10.2020 02:01





Biology, 12.10.2020 02:01

Arts, 12.10.2020 02:01

Mathematics, 12.10.2020 02:01



History, 12.10.2020 02:01

Spanish, 12.10.2020 02:01



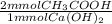 = 15.46 mmol CH₃COOH
= 15.46 mmol CH₃COOH


