Chemistry, 12.06.2021 17:50 Jenifermorales101
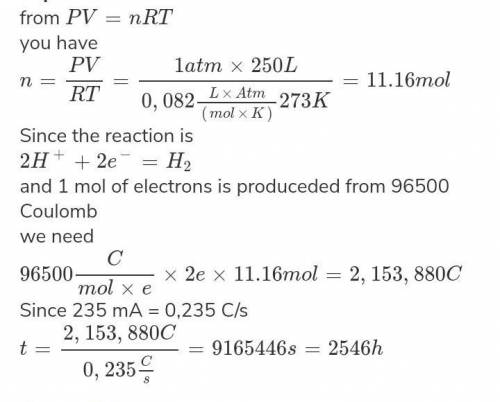
In the electrolysis of water, how long will it take to produce 75.00 L of H2 at 1.0 atm and 273 K using an electrolytic cell through which the current is 205.0 mA

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1

Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2

Chemistry, 23.06.2019 14:30
When does phenolphthalein turn pink? in the presence of a base in the presence of an acid when it is in a neutral solution when it is reacting with a metal
Answers: 1
You know the right answer?
In the electrolysis of water, how long will it take to produce 75.00 L of H2 at 1.0 atm and 273 K us...
Questions

World Languages, 09.02.2021 01:00



Mathematics, 09.02.2021 01:00

Medicine, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00

History, 09.02.2021 01:00



Mathematics, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00


History, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00




Mathematics, 09.02.2021 01:00