
Chemistry, 12.06.2021 19:30 myaaa13754
During an exothermic reaction, H for the reactants was 890 kJ/mol. Which of the following statements is correct about the H for the products and the comparison of the energy in bonds?
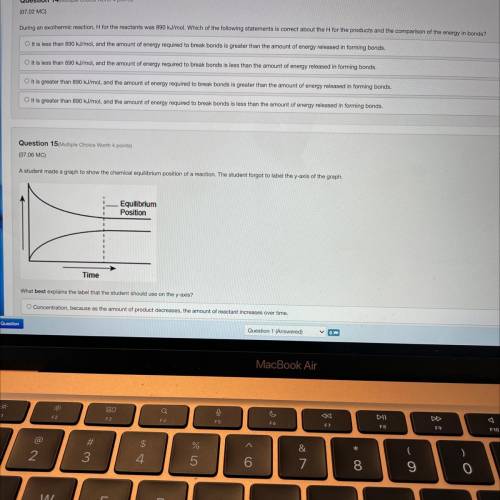

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2


Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
During an exothermic reaction, H for the reactants was 890 kJ/mol. Which of the following statements...
Questions

Mathematics, 19.04.2021 14:00

English, 19.04.2021 14:00

English, 19.04.2021 14:00



English, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00

English, 19.04.2021 14:00

English, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

English, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00


Chemistry, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00



