
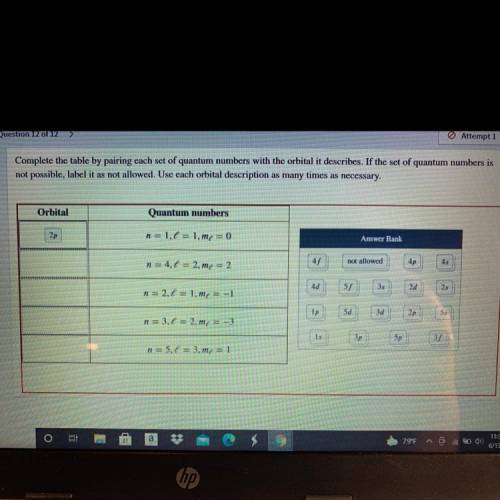
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set of quantum numbers is
not possible, label it as not allowed. Use each orbital description as many times as necessary.
Orbital
Quantum numbers
2p
n = 1.6 = 1.me = 0
Answer Bank
n = 4.1 = 2.m = 2
45
not allowed
4p
45
4d
n = 2.1 = 1.mx = -1
55
35
2d
25
Ip
5d
3d
2p
55
n = 3,6 = 2.mx = -3
1s
3p
5p
35
n = 5.0 = 3.m4 = 1
7
O
Bi
11:36 PM

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science)
Answers: 1

Chemistry, 23.06.2019 17:00
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1
You know the right answer?
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set...
Questions

Mathematics, 11.03.2021 01:20




Mathematics, 11.03.2021 01:20

Mathematics, 11.03.2021 01:20

Arts, 11.03.2021 01:20

Mathematics, 11.03.2021 01:20



Mathematics, 11.03.2021 01:20

History, 11.03.2021 01:20

History, 11.03.2021 01:20

Mathematics, 11.03.2021 01:20

Mathematics, 11.03.2021 01:20

Arts, 11.03.2021 01:20

Mathematics, 11.03.2021 01:20

Spanish, 11.03.2021 01:20




