
Chemistry, 15.06.2021 01:00 crobinson7206
Stomach acid is approximately 0.10 M HCl. How many mL of stomach acid can be neutralized by one regular antacid tablet that contains 500 mg of solid CaCO3 (100.09 g/mol)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
Stomach acid is approximately 0.10 M HCl. How many mL of stomach acid can be neutralized by one regu...
Questions



History, 15.11.2019 03:31

Spanish, 15.11.2019 03:31


Arts, 15.11.2019 03:31

English, 15.11.2019 03:31


History, 15.11.2019 03:31

English, 15.11.2019 03:31



History, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31


Geography, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31



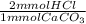 = 10 mmol HCl
= 10 mmol HCl


