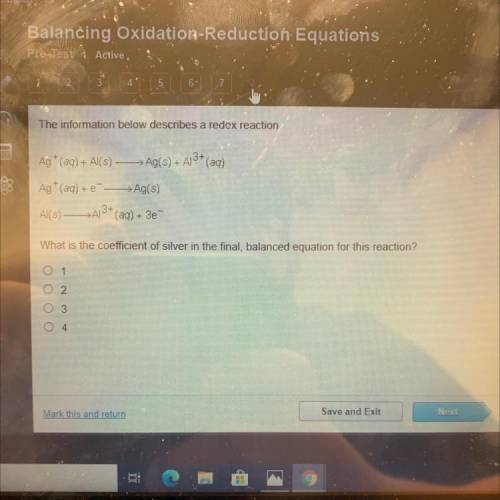
The information below describes a redox reaction.
Ag+ (aq) + Al(s) —>Ag(s) + A13+ (aq)
Ag+...

Chemistry, 15.06.2021 03:50 rubyhart522
The information below describes a redox reaction.
Ag+ (aq) + Al(s) —>Ag(s) + A13+ (aq)
Ag+ (aq) + --> Ag(s)
Al(s) - >A3+ (aq) + 3e
What is the coefficient of silver in the final, balanced equation for this reaction?
0 1
02
0 3
O 4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2



Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Questions


Mathematics, 15.04.2021 01:00



Mathematics, 15.04.2021 01:00


English, 15.04.2021 01:00








Mathematics, 15.04.2021 01:00


SAT, 15.04.2021 01:00






