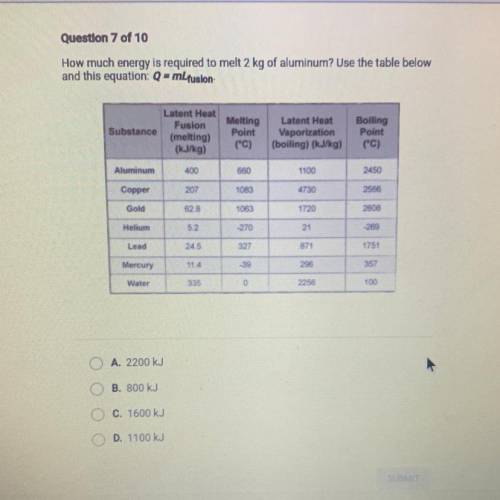
Question 7 of 10
How much energy is required to melt 2 kg of aluminum? Use the table below
an...

Chemistry, 15.06.2021 20:00 lanaiheart7
Question 7 of 10
How much energy is required to melt 2 kg of aluminum? Use the table below
and this equation: Q- mLfusion
Substance
Latent Heat
Fusion
(melting)
(kJ/kg)
Melting
Point
(°C)
Latent Heat
Vaporization
(boiling) (kJ/kg)
Boiling
Point
("C)
Aluminum
400
660
1100
2450
Copper
207
1083
4730
2566
Gold
628
1063
1720
2808
Helium
5.2
-270
21
-269
Lead
245
327
871
1751
Mercury
11.4
-39
295
357
Water
335
0
2258
100
A. 2200 kJ
B. 800 kJ
C. 1600 kJ
D. 1100 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Questions

Mathematics, 03.11.2020 08:30

French, 03.11.2020 08:30

Mathematics, 03.11.2020 08:30

Mathematics, 03.11.2020 08:30



Mathematics, 03.11.2020 08:30



English, 03.11.2020 08:30


Spanish, 03.11.2020 08:30


SAT, 03.11.2020 08:30

Mathematics, 03.11.2020 08:30



Mathematics, 03.11.2020 08:30

English, 03.11.2020 08:30



