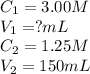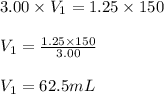Chemistry, 15.06.2021 20:30 lanettejohnson355
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solution is 150 mL. What volume of a 1.25 M solution could be made from the stock solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3


Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solutio...
Questions

Mathematics, 13.10.2020 05:01


English, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01



Mathematics, 13.10.2020 05:01



Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01



History, 13.10.2020 05:01

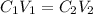 ....(1)
....(1)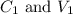 are the concentration and volume of stock solution.
are the concentration and volume of stock solution.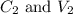 are the concentration and volume of diluted solution.
are the concentration and volume of diluted solution.