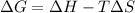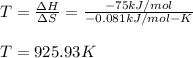Chemistry, 15.06.2021 23:40 zahraalshagawa02
For a reaction, Delta * H ^ 0 = - 75KJ / m * o * l and triangle S^ 0 =-0.081KJ/(K* mol) . At what temperatures is this reaction spontaneous?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
For a reaction, Delta * H ^ 0 = - 75KJ / m * o * l and triangle S^ 0 =-0.081KJ/(K* mol) . At what te...
Questions

Mathematics, 04.05.2021 15:00

History, 04.05.2021 15:00

Mathematics, 04.05.2021 15:00



Advanced Placement (AP), 04.05.2021 15:00

Mathematics, 04.05.2021 15:00



Computers and Technology, 04.05.2021 15:00


Chemistry, 04.05.2021 15:00

English, 04.05.2021 15:00

Mathematics, 04.05.2021 15:00

Chemistry, 04.05.2021 15:00

Mathematics, 04.05.2021 15:00




Mathematics, 04.05.2021 15:00