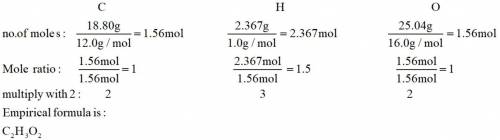Chemistry, 16.06.2021 04:00 mathiscool51
Analysis of a sample of a compound composed of carbon, hydrogen, and oxygen shows that the sample contains 18.80 g of C, 2.367 g of H, and 25.04 g of O. The properties of the compound suggest that the molar mass should be 59.04 g/mol. How many carbon atoms are there in one molecule of the compound

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Analysis of a sample of a compound composed of carbon, hydrogen, and oxygen shows that the sample co...
Questions




English, 25.02.2022 09:10

Health, 25.02.2022 09:20


Biology, 25.02.2022 09:20

Biology, 25.02.2022 09:20


Mathematics, 25.02.2022 09:20

Mathematics, 25.02.2022 09:20





Biology, 25.02.2022 09:20

English, 25.02.2022 09:20

English, 25.02.2022 09:20


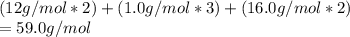
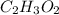 .
.