
Calculate the total amount of energy required in calories to convert 50.0 g of ice at 0.00 degrees Celsius to steam at 100. degrees Celsius.
Specific heat capacity of water is 1.00 cal/g OC
Hfusion = 80 cal/g OC and Hvap = 540 cal/g OC
Write the complete equation you will use.
Substitute the values in the equation in step 1.
Report the math answer with 3 sig figs and the correct unit.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Calculate the total amount of energy required in calories to convert 50.0 g of ice at 0.00 degrees C...
Questions

Mathematics, 27.09.2019 01:30

Chemistry, 27.09.2019 01:30


History, 27.09.2019 01:30

Health, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30

Chemistry, 27.09.2019 01:30


Biology, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30


Mathematics, 27.09.2019 01:30


Mathematics, 27.09.2019 01:30



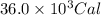
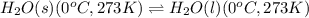
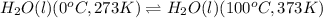
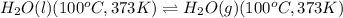
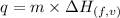 ......(i)
......(i)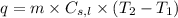 ......(ii)
......(ii)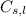 = specific heat of solid or liquid
= specific heat of solid or liquid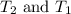 are final and initial temperatures respectively
are final and initial temperatures respectively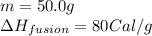
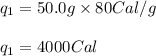
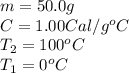
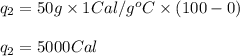
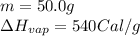
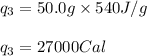
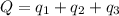
![Q=[(4000)+(5000)+(27000)]Cal=36000Cal=36.0\times 10^3Cal](/tpl/images/1376/1646/a9c1b.png)


