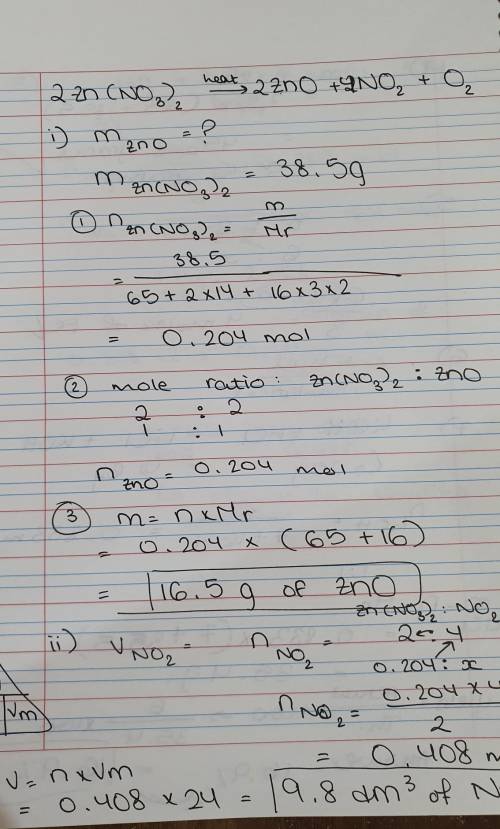When zinc nitrate is heated, zinc oxide, nitrogen dioxide(NO2) and oxygen gas are
produced.
i...

Chemistry, 16.06.2021 16:00 megankbrown
When zinc nitrate is heated, zinc oxide, nitrogen dioxide(NO2) and oxygen gas are
produced.
i.
Calculate the mass of Zinc oxide produced if 38.5 g of zinc nitrate is heated.
ii.
Determine the volume of Nitrogen dioxide gas evolved at rtp

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
Questions

Mathematics, 22.01.2021 02:30


English, 22.01.2021 02:30




Mathematics, 22.01.2021 02:30

History, 22.01.2021 02:30

Social Studies, 22.01.2021 02:30

Mathematics, 22.01.2021 02:30

Mathematics, 22.01.2021 02:30



English, 22.01.2021 02:30


History, 22.01.2021 02:30

Mathematics, 22.01.2021 02:30



History, 22.01.2021 02:30