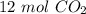Chemistry, 16.06.2021 20:40 dylanjones6996
6CO2 + 6H20 --> C6H12O6 + 602 What is the total number of moles of CO2 needed to make 2 moles of CH1206?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
6CO2 + 6H20 --> C6H12O6 + 602
What is the total number of moles of CO2 needed to make 2 moles of...
Questions

Mathematics, 04.12.2020 01:00



Mathematics, 04.12.2020 01:00

History, 04.12.2020 01:00

English, 04.12.2020 01:00


History, 04.12.2020 01:00

Physics, 04.12.2020 01:00



Mathematics, 04.12.2020 01:00



Mathematics, 04.12.2020 01:00

Business, 04.12.2020 01:00

Computers and Technology, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

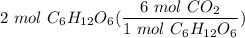 [DA] Multiply [Cancel out units]:
[DA] Multiply [Cancel out units]: