
Chemistry, 16.06.2021 22:20 erica11223344
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl2 (aq)
OA. K=[NO_ Cl]?[NO2]?[ciz]
[NO_C1]
[NO2]?[Ciz]
OB, K=
Ock=
2[NO_IC12]
2[NO2C]
OD. K=
2[NO2Cl]
2[NO2][Ciz]
OEK=
[NO2][C12]
[NO_C1]?
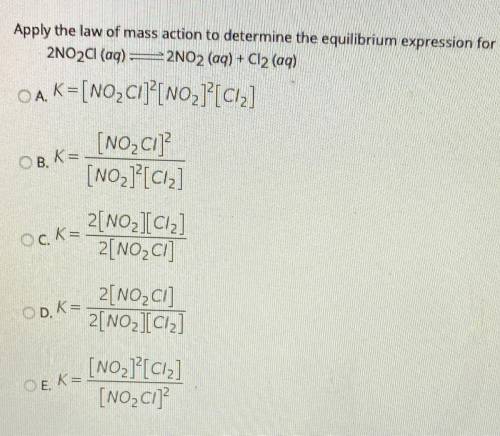

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl...
Questions




History, 13.07.2019 22:40

Health, 13.07.2019 22:40


Social Studies, 13.07.2019 22:40

Social Studies, 13.07.2019 22:40

Mathematics, 13.07.2019 22:40


Social Studies, 13.07.2019 22:40



Advanced Placement (AP), 13.07.2019 22:40

Biology, 13.07.2019 22:40


Mathematics, 13.07.2019 22:40



Business, 13.07.2019 22:40




