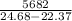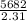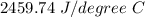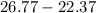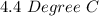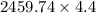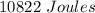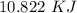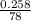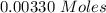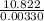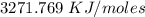Chemistry, 16.06.2021 23:10 ejcastilllo
Two experiments were conducted in a bomb calorimeter. The first one to determine the heat capacity of the calorimeter, the second the heat of combustion of the carcinogenic substance benzene (C6H6). a. In the first experiment, the temperature rises from 22.37 o C to 24.68 o C when the calorimeter absorbs 5682 J of heat. Determine the heat capacity of the calorimeter. Page 3 of 4 b. In the second experiment, the combustion of 0.258 g of benzene increases the temperature from 22.37 o C to 26.77 o C. Determine the heat of combustion for 1 mol of benzene.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Two experiments were conducted in a bomb calorimeter. The first one to determine the heat capacity o...
Questions

Mathematics, 30.08.2020 23:01


Mathematics, 30.08.2020 23:01

Mathematics, 30.08.2020 23:01

Mathematics, 30.08.2020 23:01



History, 30.08.2020 23:01

Arts, 30.08.2020 23:01


Physics, 30.08.2020 23:01

Spanish, 30.08.2020 23:01

Mathematics, 30.08.2020 23:01


Mathematics, 30.08.2020 23:01




Mathematics, 30.08.2020 23:01

Mathematics, 30.08.2020 23:01