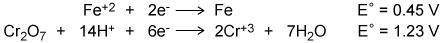Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell p...
Questions

Mathematics, 29.01.2020 19:57

Chemistry, 29.01.2020 19:57





Chemistry, 29.01.2020 19:57

Chemistry, 29.01.2020 19:57

Physics, 29.01.2020 19:57

History, 29.01.2020 19:57

Mathematics, 29.01.2020 19:57

Physics, 29.01.2020 19:57

Biology, 29.01.2020 19:57

Mathematics, 29.01.2020 19:57

Mathematics, 29.01.2020 19:57

Mathematics, 29.01.2020 19:57


Mathematics, 29.01.2020 19:57


French, 29.01.2020 19:57