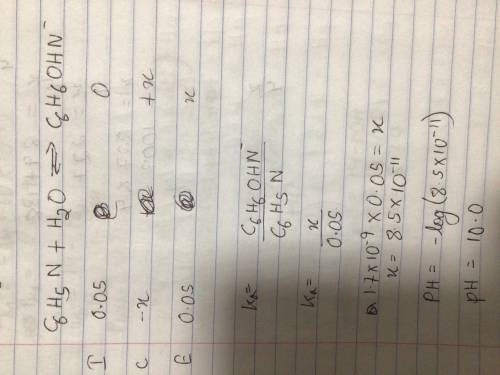Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2


Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
Find the pH of 0.05M Pyridine C 6 H 5 N solution. Know the base dissociation constant K C6H5N = 1.7....
Questions


History, 12.04.2021 21:40

Social Studies, 12.04.2021 21:40




Mathematics, 12.04.2021 21:40

Mathematics, 12.04.2021 21:40

Chemistry, 12.04.2021 21:40

Chemistry, 12.04.2021 21:40

Biology, 12.04.2021 21:40



Mathematics, 12.04.2021 21:40



Mathematics, 12.04.2021 21:40

Biology, 12.04.2021 21:40

History, 12.04.2021 21:40

Social Studies, 12.04.2021 21:40