Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
if 600.0 grams HF reacts with excess SiO2, how many grams of H2O are produced? Use the following mol...
Questions

English, 19.02.2021 22:40


English, 19.02.2021 22:40




History, 19.02.2021 22:40

Mathematics, 19.02.2021 22:40

Mathematics, 19.02.2021 22:40


Mathematics, 19.02.2021 22:40

Mathematics, 19.02.2021 22:40

Mathematics, 19.02.2021 22:40

Mathematics, 19.02.2021 22:40







 ......(1)
......(1)
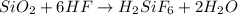
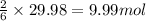 of water
of water