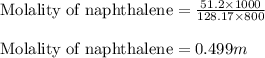Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1
You know the right answer?
Calculate the molality of a solution that contains 51.2 g of naphthalene, C10H8, in 500 mL of carbon...
Questions









Mathematics, 30.03.2020 18:56


Biology, 30.03.2020 18:56




Health, 30.03.2020 18:56

English, 30.03.2020 18:56

English, 30.03.2020 18:56

Mathematics, 30.03.2020 18:57

Mathematics, 30.03.2020 18:57

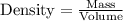 ......(1)
......(1)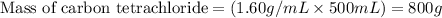
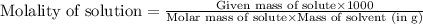 .....(2)
.....(2)