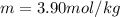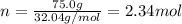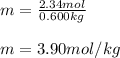Chemistry, 18.06.2021 02:50 haileyrae187
Calculate the molality of a solution that contains 75.0-grams of methyl alcohol, CH3OH, dissolved in 600.0-grams of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1


Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Calculate the molality of a solution that contains 75.0-grams of methyl alcohol, CH3OH, dissolved in...
Questions

Mathematics, 30.11.2020 02:00

Biology, 30.11.2020 02:00


Mathematics, 30.11.2020 02:00



Mathematics, 30.11.2020 02:00




Mathematics, 30.11.2020 02:00

Mathematics, 30.11.2020 02:00


Mathematics, 30.11.2020 02:00





Health, 30.11.2020 02:00